Gas Chromatography with FID/TCD/MS Detectors
Gas chromatography (GC) is used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition.
- Description
| Testing Method | Gas Chromatography with FID/TCD/MS Detectors |
| Description | Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
In gas chromatography, the mobile phase (or “moving phase”) is a carrier gas, usually an inert gas such as helium or an unreactive gas such as nitrogen. Helium remains the most commonly used carrier gas in about 90% of instruments although hydrogen is preferred for improved separations. The stationary phase is a microscopic layer of liquid or polymer on an inert solid support, inside a piece of glass or metal tubing called a column (an homage to the fractionating column used in distillation). The instrument used to perform gas chromatography is called a gas chromatograph (or “aerograph”, “gas separator”).
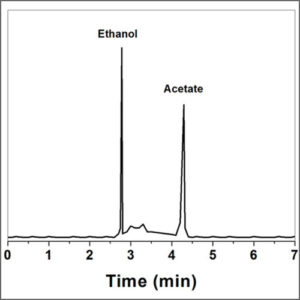
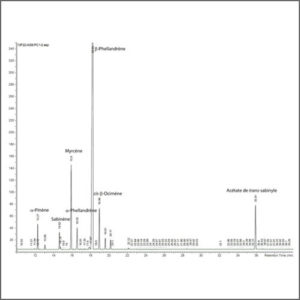
The gaseous compounds being analyzed interact with the walls of the column, which is coated with a stationary phase. This causes each compound to elute at a different time, known as the retention time of the compound. The comparison of retention times is what gives GC its analytical usefulness.
Detectors of gas chromatography:
The most commonly used detectors are the flame ionization detector (FID) and the thermal conductivity detector (TCD). Both are sensitive to a wide range of components, and both work over a wide range of concentrations. While TCDs are essentially universal and can be used to detect any component other than the carrier gas (as long as their thermal conductivities are different from that of the carrier gas, at detector temperature), FIDs are sensitive primarily to hydrocarbons, and are more sensitive to them than TCD. However, a FID cannot detect water. Both detectors are also quite robust. Since TCD is non-destructive, it can be operated in-series before a FID (destructive), thus providing complementary detection of the same analytes.
(1) Flame Ionization detector (FID), in this common detector electrodes are placed adjacent to a flame fueled by hydrogen / air near the exit of the column, and when carbon containing compounds exit the column they are pyrolyzed by the flame. This detector works only for organic / hydrocarbon containing compounds due to the ability of the carbons to form cations and electrons upon pyrolysis which generates a current between the electrodes. The increase in current is translated and appears as a peak in a chromatogram. FIDs have low detection limits (a few picograms per second) but they are unable to generate ions from carbonyl containing carbons. FID compatible carrier gasses include nitrogen, helium, and argon.
(2) Thermal Conductivity detector (TCD), this common detector relies on the thermal conductivity of matter passing around a tungsten -rhenium filament with a current traveling through it. In this set up helium or nitrogen serve as the carrier gas because of their relatively high thermal conductivity which keeps the filament cool and maintain uniform resistivity and electrical efficiency of the filament. However, when analyte molecules elute from the column, mixed with carrier gas, the thermal conductivity decreases and this causes a detector response. The response is due to the decreased thermal conductivity causing an increase in filament temperature and resistivity resulting in fluctuations in voltage.[5] Detector sensitivity is proportional to filament current while it is inversely proportional to the immediate environmental temperature of that detector as well as flow rate of the carrier gas.
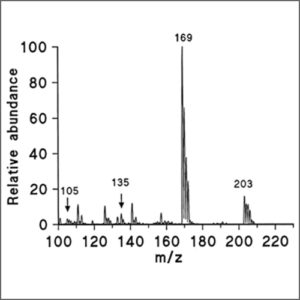
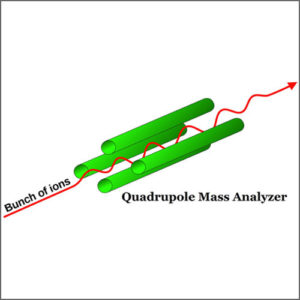
(3) Mass spectrometer detector (MS), also called GC-MS, is highly effective and sensitive, even in a small quantity of sample. The most common MS detector is the quadrupole mass spectrometer, referred to by the Hewlett-Packard (now Agilent) with trade name of “Mass Selective Detector” (MSD). |
| More Information | Wikipedia: Gas Chromatography |