X-ray Photoelectron Spectroscopy (XPS)
XPS can measure the elemental composition, empirical formula, chemical state and electronic state of the elements at the surface of a material.
- Description
| Testing Method | X-ray Photoelectron Spectroscopy (XPS) |
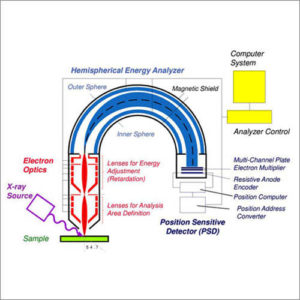
| Description | X-ray photoelectron spectroscopy (XPS) is a surface sensitive analytical technique that measures the binding energy of electrons in atoms and molecules on the surface of a material. XPS is a useful measurement technique because it not only shows what elements are within a film but also what other elements they are bonded to. This means if you have a metal oxide and you want to know if the metal is in a +1 or +2 state, using XPS will allow you to find that ratio. However at most the instrument will only probe ~12nm into a sample. XPS spectra are obtained by irradiating a material with a beam of X-rays while simultaneously measuring the kinetic energy and number of electrons that escape from the top 1 to 12 nm of the material being analyzed. XPS requires ultra-high vacuum (UHV) conditions.
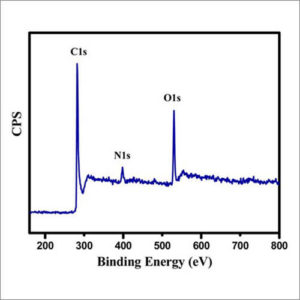
A typical XPS spectrum is a plot of the number of electrons detected (sometimes per unit time) (Y-axis, ordinate) versus the binding energy of the electrons detected (X-axis, abscissa). Each element produces a characteristic set of XPS peaks at characteristic binding energy values that directly identify each element that exists in or on the surface of the material being analyzed. These characteristic spectral peaks correspond to the electron configuration of the electrons within the atoms, e.g., 1s, 2s, 2p, 3s, etc. The number of detected electrons in each of the characteristic peaks is directly related to the amount of element within the XPS sampling volume. To generate atomic percentage values, each raw XPS signal must be corrected by dividing its signal intensity (number of electrons detected) by a “relative sensitivity factor” (RSF), and normalized over all of the elements detected. Since hydrogen is not detected, these atomic percentages exclude hydrogen. To count the number of electrons during the acquisition of a spectrum with a minimum of error, XPS detectors must be operated under ultra-high vacuum (UHV) conditions because electron counting detectors in XPS instruments are typically one meter away from the material irradiated with X-rays. This long path length for detection requires such low pressures.
XPS is a surface chemical analysis technique that can be used to analyze the surface chemistry of a material in its’ “as received” state, or after some treatment, for example: fracturing, cutting or scraping in air or UHV to expose the bulk chemistry, ion beam etching to clean off some of the surface contamination, exposure to heat to study the changes due to heating, exposure to reactive gases or solutions, exposure to ion beam implant, exposure to ultraviolet light.
XPS is also known as electron spectroscopy for chemical analysis (ESCA). The detection limits of XPS are in the parts per thousand range (1,000 PPM) for most of the elements. XPS is routinely used to analyze inorganic compounds, metal alloys, semiconductors, polymers, elements, catalysts, glasses, ceramics, paints, papers, inks, woods, plant parts, make-up, teeth, bones, medical implants, bio-materials, viscous oils, glues, ion modified materials and many others. |
| More Information | Wikipedia: X-ray Photoelectron Spectroscopy |